How much salt to use for the perfect lacto fermentation salt ratio is a common question. Let’s go over how much salt to use when fermenting vegetables, and how to measure the perfect fermenting brine ratio.
The Perfect Lacto Fermentation Salt Ratio
Particular concentrations of salt pave the way for creating a happy home for lactic acid-producing bacteria. Mainly, Leuconostoc and Lactobacillus bacteria thrive in fermented vegetables.
When trying to determine the perfect lacto fermentation salt ratio, it isn’t about how salty or not salty you like your fermented vegetables. We have to focus on how salty certain microbes like it.
All the microbes needed for the fermentation process are already present on your vegetables, even after you wash them. Once you provide the proper salt concentration, bacterial succession can occur, and lactic acid can be adequately produced.

How to Measure the Perfect Fermenting Brine Ratio
To use a precise and accurate salt concentration, you must use units of mass to measure your salt.
There are two mathematical ways to create an exact percent salt concentration, and the desired salt concentration varies by type of vegetable.
You can see recommended salt concentrations in our blog, The Complete Guide to Safely Using Salt in Vegetable Fermentation.

Let’s look at 2.5% as an example
To create an exact 2.5% total salt concentration, place a bowl on a scale and tare/zero the scale. Add 2.5 grams of salt to the bowl, then add your produce and any water into that same bowl up to 100 grams. That’s a 2.5% total salt concentration.
What we do is slightly different and easily applied to variable amounts.
How We Measure the Perfect Fermenting Brine Ratio
We weigh all our produce and water, multiply that weight by 2.5%, and add the number we get in grams of salt.
This results in an approximate 2.5% salt concentration that is perfectly safe and optimal for fermentation. Also, this mathematical method can work with any total salt concentration.

lacto fermentation salt ratio example:
If we have 100 grams of produce and water, we multiply by 2.5%. 100 x 0.025 = 2.5. So we add 2.5 grams of salt. This ends up being a 2.44% total salt concentration.
To calculate the total percent salt concentration of the entire mixture, you divide the grams of salt by the total grams of the whole mixture: 2.5 grams of salt / 102.5 grams (of salt + water + produce) = 0.02439.
Move the decimal to make it a percent, and you get 2.44%
And guess what? With this method, we end up with 2.44% salt, no matter the weight of vegetables or water… if we add 2.5% salt, the resulting total salt concentration will always be 2.44%
lacto fermentation salt ratio example 2:
If we have 756 grams of cabbage and water, we multiply that by 2.5%. That equals 18.9. So we add 18.9 grams of salt.
18.9 / (756+18.9) = 0.02439
Yep. That’s 2.44%
You will only get a consistent salt concentration throughout different fermentation batches by weighing the produce and water, doing math, and then weighing out your salt.

Lacto Fermentation Salt Ratio
You might be wondering why we use the weight of water and the weight of vegetables to determine the perfect salt ratio for fermentation. All vegetables are at least 93-98% water, so you must account for the water inside the vegetables. Because of osmosis and concentration gradients, the total salt concentration includes the water in the vegetables.
Between batches and throughout the seasons, the weight of the vegetables you are fermenting will vary, because the water density of the vegetables will vary. For instance, a summer cabbage is much lighter than a winter cabbage because summer cabbages aren’t as water-dense.
Measuring Salt in Vegetable Fermentation
You may also be wondering why we should use weight to measure ingredients. Simply put, the same volume of different salts, contains different amounts of NaCl.
Depending on what brand, style, and type of salt you are using, the amount of NaCl in a volume measurement, such as a tablespoon, varies greatly.
Unrefined sea salt can be found in many different “grains,” such as flake, large grain, small grain, etc. A teaspoon of flake salt has a mass of about 1 gram. A teaspoon of small-grain Himalayan salt has a mass of about 3 grams.
If you add a teaspoon of flake salt to 100 grams of vegetables and water, you get a 0.99% salt concentration. If you add a teaspoon of Himalayan salt to 100 grams of vegetables and water, you get a 2.9% salt concentration. That’s a huge difference, and the 0.99% salt concentration is completely unsafe and probably won’t select for probiotic microbes.

Other Fermentation Factors
Accurate salt concentration, adequate time for the fermentation, and proper temperature are necessary for a healthy population of microbes to develop in vegetable fermentation.
The temperature for vegetable fermentation should be between 70-80 degrees F. However, anywhere between 60 and 90° F is acceptable. The cooler the temp, the slower the fermentation. The hotter the temp, the faster.
Once the temperature is established, the two main factors that need to be tailored to the microbes are salt concentration and length of time for the fermentation.
Suppose all of these things are accounted for appropriately. In that case, the pH of the fermentation will drop, lactic acid will build up, the microbial population will consist only of probiotic bacteria, and the vegetable matter will be preserved.
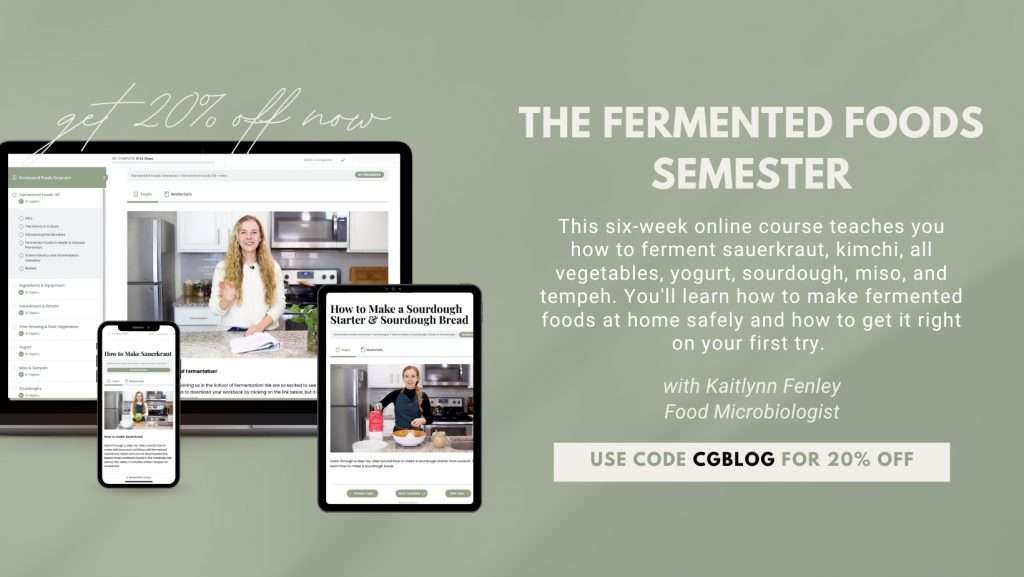
Get Started Fermenting At Home
- How to Make Old Fashioned Sauerkraut with Caraway Seeds
- Homemade Kimchi Inspired Spicy Sauerkraut Recipe
- Pepper Fermentation Recipe: Learn How to Ferment Any Type of Pepper
References:
Salt Reduction in Vegetable Fermentation: Reality or Desire?
Effects of salt concentration on Chinese sauerkraut fermentation
Survival of Escherichia coli O157:H7 in Cucumber Fermentation Brines
Frias, Juana & Martinez-Villaluenga, Cristina & Peñas, Elena. (2016). Fermented Foods in Health and Disease Prevention.


















Hello! Thanks for pointing out the typo! It’s supposed to be anaerobic. Assuming my auto correct was confused by the "an anaerobic" and thought I was repeating "an"!
Fantastic blog! I learned so much and will definitely be saving this. I only wish someone with this knowledge went into a deep dive of on kombucha brewing. Needless to say, if you branch out, I’ll be eager to read 🙂
Salinity, salt solutions, “saltiness” via taste perception, and salt concentration are all different things. Solutions and mixtures are also two different things if you’re speaking about science.
Fermented vegetables are a mixture of salt, water and vegetables. Total Salt Concentration refers to the total amount of salt present in comparison to the amount of the entire mixture (including all the salt, water and vegetables).
So salt concentration should be calculated (grams of NaCl added) divided by (the combined mass of the water, vegetables and added salt).
If you add 2.5% of the mass of vegetables and water in grams of salt, you will always end up with a 2.44% total salt concentration of the mixture. The type of vegetable is irrelevant as long as you weigh it. (except for root vegetables, that is what I have a disclosure statement within this blog about this not pertaining to root vegetable fermentation). Understanding that 1 milliliter of water weighs 1 gram and that mostly all vegetables are between 91-98% water helps this to make sense… especially when you account for the fact that the 91-98% water will diffuse in and out of the plant tissues with osmosis/concentration gradients.
Salinity (which is the concentration of salt in a homogenous solution of salt and water) is typically measured as concentration of grams of salt per kilograms of water. So to calculate you would have to add the mass of the water + the percent of the vegetable mass that is water in kg… then it’s: (the mass of the salt added in grams) divided by (the combined mass of water added + water mass of the vegetable in question in kg). The unit of measure for the result is ppt (parts per thousand), not a percent.
This is also why, if you make sauerkraut without the addition of water, you just add 2.5% of the cabbage weigh in salt. The salt draws the water out of the cabbage, and you again end up with a 2.44% total salt concentration of the mixture.
In culture media: if I wanted to make a 2.5% NaCl agar plate I would add 2.5 grams of NaCl to 97.5 grams of mixed media (solid agar, mixed in liquid water).
Nice explanation. Thank you
You’re welcome!
Thank you so much for this article. I am one that is terrible at math and i always want the science behind things… This article spells out the math so I will not have a problem with it. Science behind especially food. I brew milk kefir and keep bread starters . The science was and still is often hard to find. I have search for years about the science behind bread making With no real luck.
So thank you for the detailed explanation about the mouth and the science and the process of the science.
Thank you for your information! After seeing so many differing opinions about the amount of salt needed to ferment vegetables, this is the first source I’ve found that is backed by science. Considering the real potential for food borne illness that can come from culturing microbes found in the wild, I feel much better using your methods than just winging it.
The salt percentage is defined by the living parameters of the microorganisms involved in the fermentation process. It is also defined by the living parameters of microbes not wanted in the fermentation process.
Both. One can taste test things in stage two of fermentation, because Leuconostoc bacteria are not harmful… but allowing time for the acid to build up is what makes the fermentation preserved AND safe. Proper pH = Safety. Once you start putting utensils in the fermented mixture, you expose new microorganisms to the mix. To prevent contamination, it needs to be fully preserved with enough microbe-produced acid.
It’s not salinity, It’s total salt concentration. two different things. The trend of salt concentration and fermentation time is not linear, and there are parameters of too little salt and too much salt. It all depends on the vegetables, the vegetable microbiome, the temperature, the salinity, the fermentation vessel, seasoning and spices used. etc. It’s the environment as a whole that influences fermentation time. There is some literature on this, I use about 30-40 different microbiology textbooks when I need to reference… but mostly I do my own research and draw my own conclusions from my five years of education in Microbiology. You can try to use google scholar to find any publish papers on the matter.
Hope this helps! 🙂
Thanks for this. I’ve been fermenting my home-grown hot peppers into sriracha sauce for a few years with great success, but my cucumbers have been hit and miss, and now I know why. Whereas my 2% (salt) pepper mash is calculated simply by weighing the mashed peppers and multiplying by .02 for the proper amount of salt, it appears, according to this article, that the cucumbers AND the water must be weighed together in order to calculate the proper amount of salt for immersion fermentation. This makes sense. Previously I had been adding 2 tablespoons of pure "pickling" salt to a quart of spring water, regardless of how many cucumbers were in the batch, thus the inconsistent results.
I have a huge National Pickling cucumber patch (jungle is more like it) and they are just starting to mature to harvest size. I’ve begun a test batch with the first 8 cukes using your methodology, creating a 5% brine. For the next two batches I’ll go with a 4% and a 3% respectively, keeping meticulous records and judging the winner by taste, texture, etc. If you’d like, I’ll be happy to report my results later on. Thanks again for this refreshingly scientific approach to pickling.
I’d love to learn about your results! Glad we can offer some help for consistency with your fermentations. : )
Hello again. The experiment was a great success! I created batch after batch as the cucumbers ripened from late July through late August. The first batch was a 5% salt solution — way too salty — so I gradually reduced each successive batch down to 3%, settling on 3.5% as my favorite. I used pure pickling salt. The average fermentation time was 21 days with a maximum of 25 days, at about 72°. The cucumbers were translucent throughout, firm and crunchy, and remain so. I have only 3 jars left out of 12 batches!
I just inherited a giant cabbage, so now I have to relocate your sauerkraut recipe. Thanks again!
I do mention acetic acid formation. This happens in stage two of vegetable fermentation, and the acetic acid is produced by Leuconostoc bacteria. See the paragraph under the heading “Stage Two”
In vegetable fermentation, there aren’t really conditions that favor acetic acid over lactic acid. The formation of acetic acid is simply a checkpoint that happens when heterolactic Leuconostoc bacteria thrive. They quickly die off and then homolactic Lactobacillus spp. thrive and produce copious amounts of lactic acid. Minimal acetic acid will still be present, just overpowered by lactic acid. This is all part of bacterial succession in wild fermentation. Each group of microbes produces different byproducts.
Making vinegar (acetic acid) is a completely different process from vegetable fermentation. It requires the presence of more sugars. Using apples as an example: yeasts will metabolize the apple sugars into alcohol first. Then acetic acid bacteria convert the alcohol into acetaldehyde, then into acetic acid. This process requires a starter (aka a MOV) to ensure the presence of the right kinds of acetic acid bacteria.
Very informative article, thank you. As this ratio is for vegetables, what percentage of salt would be ideal for fermenting grains like quinoa?
You cannot lactic acid ferment quinoa the same way you ferment vegetables. You will end up with alcohol. See the most recent blog post on our home page, it’s a complete guide to using salt in fermentation.
Hi, Kaitlynn.
In our tropical climate, a common room temperature here is around 86-90 F. Hence, not possible for me to place it in a room with the ideal 70-80 F temperature range as you advise above. Can I still ferment my vegies in room temperature then?
Or should I just place it in a Fridge? For how many Days till good to be consumed with enough probiotics in it?
If such given temperature range is still possible, for how many Days till good enough to be moved to Fridge? What are the expected Differences we will see as the Results? E.g. taste, bacteria types, bacteria volume, benefits, etc.
Looking forward to your answers. Thanks!
You can still ferment at room temperature! Two things are likely to happen:
1. Softer fermented vegetables, like cucumber and zucchini, may become soggy because of intense microbial action. Leuconostoc spp. and Lactobacillus spp. LOVE warm temperatures, and are VERY active at temps between 85-100 F. You may find that even fermented cabbage isn’t as crunchy.
2. Because of the intense microbial action, things will ferment faster. Generally, everything is about 5-7 days faster at your temperature range. So just knock about 7 days off the timelines I provide, then put it in the fridge.
This is all excellent information . I love getting to the how and why of things. I do have a question on timing. Every source I find on kimchi making calls for a shorter ferment time. Is kimchi a different type of fermentation i.e. not a brine? It also doesn’t seem to be a vinegar process. Does the initial salt soak help kill off pathogens so it is safer?
Fantastic explanation of the succession stages. I am pro wild fermentation and would love your thoughts on the use of veggie starters and how they would effect the 3 stages of fermentation.
Thank you for the great and helpful information. If I’m using Celtic sea salt that has a high moisture content, should I try it before I measure it out? It seems like the moist will alter the final salt concentration.
Celtic sea salt does have about 10% residual moisture, but residual moisture isn’t just water… it’s other minerals too. The residual moisture content in Celtic and Sel Gris salts will change the total salt concentration slightly, but not by an amount that’s relevant to food safety. You can just measure it using the method outlined in this blog and it will work fine.
Very informative and well explained article. I am new to fermentation and find salt amounts recommended online very confusing. Your science and research based explanation is reassuring. I have to buy a kitchen scale that will measure the amounts you recommend, I think I saw the scales you use somewhere in your blog. I have a question about lactic acid and the effect it might have on arthritis. Is it safe to eat fermented vegetable for an arthritic? And also does it affect blood pressure or diabetes. Thank you
Hey there,
I’m glad you enjoyed the blog post! Lactic acid consumed through food shouldn’t influence arthritis, but you need to consult your health care provider if you have concerns about introducing new foods to your diet. Fermented vegetables contain sodium and therefore do influence blood pressure.
Thank you for your answer regarding arthritis, and possible influence of salt fermented vegetables on blood pressure. Maybe I can wash off the salt from fermented vegetables!!! Thank you
Is there an exact optimal salt concentration for fermenting cabbage? I usually see ranges of 2 to 2.5%, but with a digital scales i can accurately measure salt in grams to at least 2 decimal points, and my cabage as well. Do given i can do this, what is the optimal salt concentration to 2 decimal points? By optimal i mean the crunchiest, least salty tasting, most laden with the right nutrients such as lactic acid once the 3 + weeks of fermentation is done.
Great, informative blog! Question: Since most of the salt is rinsed from kimchi, and it only takes 1-2 days, is it fermented?
Thank You
I think you’re talking about traditional Korean kimchi which is a completely different fermentation process, with different desired outcomes. “Most of the salt” is NOT rinsed from the cabbage when making Korean kimchi. Traditionally, Nappa cabbage is covered with 10% salt for 24 hours before rinsing and using to make kimchi. Because of something called concentration gradients, about half of that salt remains within the cabbage leaves after rinsing. Korean kimchi is usually refrigerated after 3-5 days of fermentation at room temperature because the effervescence from stage 2 of fermentation is a desired sensory quality.
My sauerkraut with apple has dried, there is now brime over the weight. It is in week three. Did not use your recipe on this one. Should I throw it out?
if it did not stay below the brine, I’d throw it out. I cannot offer much advice on other people’s recipes.
My question is, I’ve followed your timeline and salt concentration, as I’ve typically use 2% and and a week at room temp (forc me that’s between 73-75F). Using your parameters of 2.5% and at least 21 days I find that the vegetables are extremely acidic to the point that they are no longer enjoyable, and I like acidic food! But it’s too acid, like they are steeped in pure vinegar except they don’t taste like vinegar just acid. At least they are crisp! Is this normal? are they supposed to be this strongly acidic? and maybe it’s just a matter that I’m not used to eating food that acid? How should a vegetable taste using your technique?
Taste is arbitrary and different for every person. So taste is not a reliable way to measure the success of my fermentation techniques. The vegetables should taste quite tart and sour… I suggest testing the pH. Fermented foods should always be around 3.5 to 3.7 pH. If you don’t like food in this pH range, then you don’t like acidic food. Quality sauerkraut, pickles, and other fermented vegetables should always be this acidic.
I am so appreciative of this article and really excited to have found your blog! I have been fermenting cabbage into sauerkraut for a while now and just started with shishitos from my garden. So far so good!
I do have a slightly technical question about brine and salt concentrations. I first learned about “equilibrium” brining with meats. When using salt and sugar one used the water weight plus the weight of the water in the meat. Not an exact science but the government does provide some good whole muscle numbers. I have used the shortcut of water plus the water in the meat (say natural not pre brined or injected chicken breast is 60% water as an example though I don’t remember the exact number)…you would take 100% of the water weight and 60% of the chicken weight and multiply by your goal percentages.
So yes it is a shortcut that slightly under salts due to not taking the weight of the salt into account. But close enough since it is in the fridge.
When using pink salt (nitrites) you need to be more exact and solve for the concentration you want with a bit of algebra. I really don’t do these much more risky brines because of the precision needed.
So that was long winded…now my question. Technically shouldn’t you be weight the water in the vegetables and not their total weight? Of course even if we have averages of 93-98% it will vary among specimens, and it is so close to 100% as not to matter. Especially since you will get slightly more salt with your approach. Not a safety issue. Just curious if the correct approach conceptually is to account for the water, the water in the veg, and the weight of the salt such that the weight of the salt is your target percent?
I love an inquisitive comment!
Yes, you are correct. For an exact measure of brine salinity even for vegetables, you would calculate using 100% of the water weight and the % water weight of the vegetable, not the total weight, as with your chicken example. That’s the correct approach to calculate salinity because salinity is the total amount of salt dissolved in an amount of water.
But yes, as you said, vegetable water concentration is so close to 100% that the distinction is basically negligible.
That’s why I’m not teaching here how to calculate salinity, I’m teaching % composition of the total mixture (total w/w salt concentration… % of salt in the total mixture, including solid matter)
I like to teach this simpler way to achieve total w/w % of salt, instead of teaching salinity. This calculation is much easier than calculating salinity. It’s a lot easier for people to understand and allows people to achieve safety and consistency without scaring them off with too many math steps.
Thank you for your article. One commenter (hopefully the thread I am replying on) was asking about specifically calculating salinity using the water weight and the water weight of the vegetables. You point out that you don’t use your articles formula for root veggies—and I’m curious what you do for those types of veggies. Instead do you calculate the salinity as added salt divided by (water in the veggies + added water + added salt)?
I’d love to read more about this from the science side. Would you please recommend a book/article or two that I could look at?
Thank you!!
I use a completely different method for root vegetables. I call it wild heirloom culturing. Here are two recipes Easy Fermented Quick Pickled Carrots and Fermented Quick Pickled Red Onions
How about measurements in terms normal people can understand.
“normal” people are perfectly capable of using a kitchen scale to measure ingredients. It’s not difficult.
Again, so grateful for the attention and intention in these blogs. BIG gratitude!!!
I am curious about using water kefir (grains or solution) to ferment vegetables. Is this possible? If possible, how would you think about that? Or is this completely nonsensical from a microbiology standpoint?
I found only a little on the internet…. doesn’t seem to be a lot out there. Would love to hear your thoughts…
Thank you for this clear explanation of salt, percentages, salinity etc. I appreciate the science behind everything and like to try and understand it. I loved my microbiology class before nursing school- I should have studied it! What you do fascinates me as I love food, the history and science of it and microbiology. How lucky for you to have found such an interesting career!
I’ve got a few ferments going with mostly peppers and just a little garlic and they are all done at 4% salinity based on the weight of the water and vegetables combined. I’m worried to maybe this is too much salt and that the fermentation won’t start. Am I okay and it’s just going to take longer? If not should I dilute the brine by pouring some out and adding back distilled water? Advice from pepper groups is all over the map and more anecdotal than scientific. What would be the ideal percentage for lacto fermenting chili peppers? Thanks!
Peppers are good anywhere between 3% and 6% total salt. So they should be great. You should see them bubbling in the first 3-5 days of fermentation. If the peppers are extremely high on the scoville chart (like ghost peppers, Carolina reaper etc.) very high capsaicin inhibits fermentation. I teach pepper fermentation in this recipe Pepper Fermentation Recipe: Learn How to Ferment Any Type of Pepper
With a 2.5% brine (based on veg + water weight), after 10 days I get a pH of 5 to 5.2 (carrots, cucumbers…). Before eating, I check the pH, and I have to add more salt till I get a 4.6.
No mold, no yeast, but still at 5.2 pH.
Any ideas? Is it safe to eat it at 5.2 pH?
Do you check the pH?
I’m not sure what you’re explaining here. You don’t add more salt to make it acidic. Once you start the fermented vegetables they should get bubbly and start getting more acidic within 2-3 days. I do not lacto ferment carrots, because they don’t ferment well.
It sounds like you might not be measuring your salt correctly. 5.2 is not a safe pH.
I always check pH, with a calibrated pH meter when testing and writing my blog recipes.
Hola Kaitlynn, mi nombre es Adriana y te sigo desde hace mucho tiempo. Has inspirado mi trabajo y ahora tengo un proyecto de fermentos. Estoy en Merida Yucatan en Mexico. Quiero hacerte dos preguntas:
1.- la temperatura aqui es alta y en ocaciones la fermentación es muy rapida y pronto dejan de estar crujientes los vegetales. Sabes si hay alguna hoja o semilla que le pueda añadir para que lo conserve crujiente?
2.- me inspiraste y compré un barril de 100 kilos de roble acá en mexico pero pronto dejo de funcionar. Es posible me puedas compartir la información de donde puedo adquirir como los que ustedes usaban para traer yo a mexico?
Bendigo y Agradezco tu enseñanza y el amor compartido por las bacterias y sus divinas consecuencias
I love all your interesting ferments. I believe i am missing something in this post. You say the combination of vegetable and water to 100 grams.
What is the ratio for them?
You could have 90 grams water and 10 grams veg or visa versa. Producing 2 very different results.
What am I missing? Thanks
I want this blog to focus specifically on calculating how much salt to use, so I did not include information on the other nuances of developing a fermentation recipe from scratch.
The calculation amounts I presented here are only mathematical examples of determining salt. Generally The ratio of vegetables to water shouldn’t fall below 50:50. It’s understood that you fill the vessel with vegetables and then top with water to determine the combined weight of vegetables and water.
Thanks for this article and formulas. I have what I hope is a simple and not a silly question. Do you add the salt to the container with the veggies and water after weighing, as opposed to dissolving the salt in just the water? I ask, because some recipes I have seen say to dissolve X amount of salt into X amount of water and then add to jar.
Thanks!
not a silly question! Either way works, as long as the amount of salt you are adding is calculated with the weight of water and vegetables.